Introduction
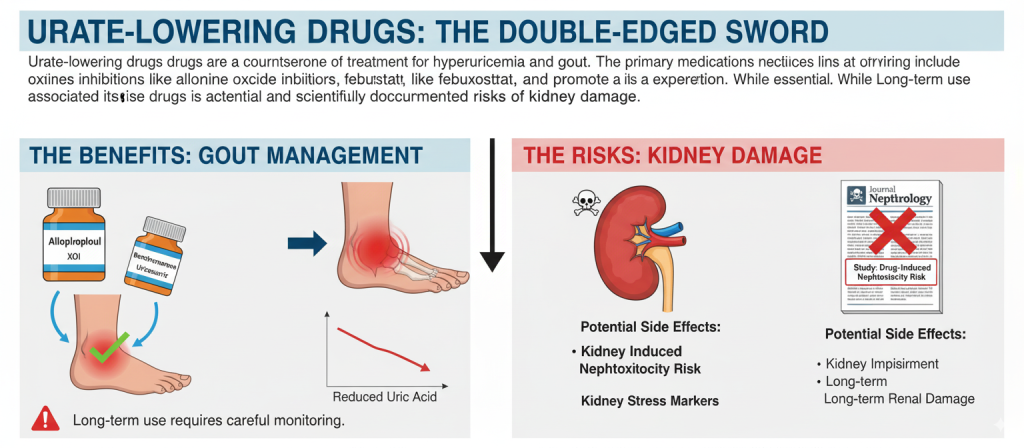
Urate-lowering drugs are a cornerstone of treatment for hyperuricemia and gout. The primary medications include xanthine oxidase inhibitors like allopurinol and febuxostat, which reduce uric acid production, and uricosuric agents like benzbromarone, which promote its excretion. While essential for managing gout, the long-term use of these drugs is associated with potential and scientifically documented risks of kidney damage.
The Kidney’s Burden: Why Gout Medications Pose a Risk
The majority of urate-lowering drugs and their metabolic byproducts are primarily excreted by the kidneys. Long-term medication use inherently increases the kidneys’ workload as they must continuously process these compounds. This is particularly concerning for patients with pre-existing renal impairment, where reduced function can lead to drug accumulation, significantly amplifying the risk of further kidney injury.
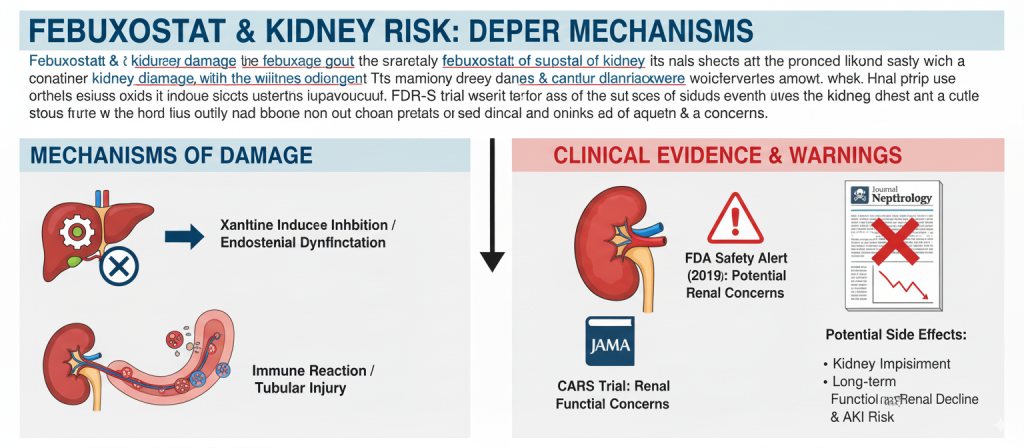
Mechanisms of Kidney Damage: The Evidence for Each Drug
Allopurinol
Allopurinol and its active metabolite, oxypurinol, are cleared by the kidneys. In patients with decreased renal function, oxypurinol can accumulate, triggering severe hypersensitivity reactions that lead to acute interstitial nephritis (AIN) and fibrosis. Studies published in journals like the New England Journal of Medicine have identified allopurinol as a common cause of drug-induced AIN. It is estimated that 0.5% to 1% of long-term users experience some form of kidney damage, a risk that is notably higher in elderly patients and those with chronic kidney disease (CKD).
Febuxostat
While febuxostat is primarily metabolized by the liver, some of its metabolites are still excreted renally. Potential mechanisms for kidney damage include inhibition of xanthine oxidase affecting renal endothelial function or the induction of immune reactions causing tubular injury. The U.S. Food and Drug Administration (FDA) issued a safety alert in 2019 regarding an increased risk of cardiovascular events and noted potential renal concerns. Furthermore, the CARES trial, published in JAMA, found that patients in the febuxostat group had a slightly higher rate of renal function decline compared to the allopurinol group and a significant association with acute kidney injury (AKI).
Benzbromarone
Benzbromarone works by inhibiting the reabsorption of uric acid in the renal tubules. This sudden surge in uric acid excretion can lead to the deposition of urate crystals in the tubules, causing obstructive nephropathy or kidney stones (nephrolithiasis). The European Medicines Agency (EMA) has highlighted its risks, and studies from Japan have shown that approximately 1.5% of long-term benzbromarone users develop kidney stones or tubular dysfunction.
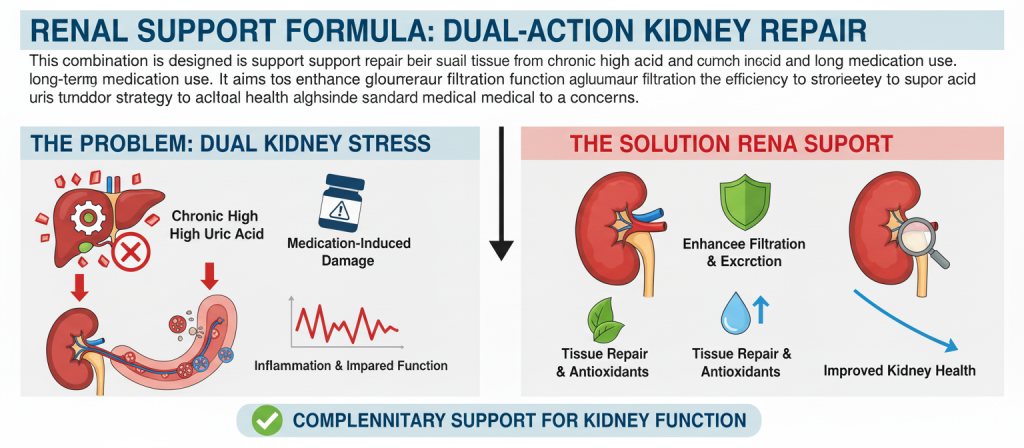
A Supportive Approach: The Role of Nutritional Intervention
For individuals concerned about the long-term impact of high uric acid and its medications on kidney health, nutritional supplements can offer a supportive role. BISPIT Compound Oyster Peptide Tablets is a dietary supplement specifically designed to support renal function in this context.
Its formula combines several bioactive ingredients for a synergistic effect:
Sea Cucumber: Known for its anti-inflammatory and tissue-repair properties.
Ginseng Saponins (from Ginseng Extract): May help improve renal microcirculation and cellular metabolism.
Oyster Peptides: Rich in taurine and zinc, which help mitigate oxidative stress on renal tubules.
This combination is designed to support the repair of kidney tissue from the dual insult of chronic high uric acid and long-term medication use. It aims to enhance glomerular filtration function and the efficiency of uric acid excretion, providing a complementary strategy to support overall kidney health alongside standard medical treatment.
References:
Food and Drug Administration (FDA). (2019). FDA adds Boxed Warning for increased risk of death with gout medicine Uloric (febuxostat). FDA Drug Safety Communication.
White, W. B., Saag, K. G., Becker, M. A., et al. (2018). Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. The New England Journal of Medicine, 378(13), 1200-1210. (Primary publication of the CARES Trial).
Vargas-Santos, A. B., & Neogi, T. (2018). Allopurinol and Chronic Kidney Disease: To Use or Not to Use? The New England Journal of Medicine, 378(13), 1251-1252.
European Medicines Agency (EMA). (2014). Assessment report for benzbromarone containing medicinal products. EMA/CHMP/18868/2014.
